 |
| Among the earliest life reconstructions of Megaloceros is this small illustration from an 1854 issue of the periodical Die Gartenlaube. Its presence may have been influenced by the Crystal Palace Megaloceros covered in the same magazine earlier that year. Although a relatively unremarkable restoration in most respects, it shows the same regal pose that has stereotyped portrayals of Megaloceros for nearly two centuries. If you've drawn or sculpted Megaloceros at some point, chances are it was standing somewhat like this. |
An upshot of Megaloceros always being 'around' is that it's easy to overlook some of the cooler aspects of its palaeobiology. Its size and impressive antlers have become the principle takehome about its existence, such that other facts about its anatomy and ecology are ignored or forgotten. Recently, I've had cause to dig into the palaeobiology and research history of Megaloceros and, wow, what an animal. Cervids are genuinely fascinating anyway: charismatic, complex and awesome animals which, behind their soft, doleful eyes, have a physiology and life history evolved in partnership with Michael Bay. And it's within this context of deer being awesome, unusual animals that we can view Megaloceros as a kind of "superdeer": a species that represents the known extreme of many trends in cervid evolution, and that should be known for much more than its size and antler spread.
Giant deer 101
M. giganteus is a well-known taxon represented by thousands of fossils spread across a wide but latitudinally-restricted distribution through Pleistocene and Holocene deposits of Europe and Asia (Lister 1994; Geist 1999; Vislobokova 2012; Lister et al. 2019). Like many Ice Age species, its distribution was continually shrinking and expanding in response to glacial growth and it never occupied the full extent of its known range at any one time. As with the mastodons discussed here recently, M. giganteus was an Ice Age species that avoided the cold. Although an adaptable animal that seems to have lived in a range of environments, Megaloceros appears to have thrived mostly during interglacials when the well-vegetated, herbaceous habitats it preferred were at their broadest (Vislobokova 2012). It lived in largely open, flat and well-watered settings termed ‘boreal parkland’ or ‘boreal steppe-woodland’, which were situated between cold steppe-tundra in the north and warmer, arid regions to the south. This parkland habitat provided Megaloceros with a diversity of grazing and browsing opportunities thanks to the presence of pine and spruce trees, as well as dense shrubs and herbs. The precise habitat preferences of Megaloceros changed over time and is reflected in antler shape: early subspecies had smaller, more erect antlers and seems to have preferred slightly more forested settings, for example (Lister 1994). Access to good quality food and water were likely critical to Megaloceros life histories due to the nutritional demands of growing those huge antlers each year (Geist 1999), and their need for highly productive plant communities likely prevented them from attaining the wider geographic ranges of other famous Pleistocene megafauna (Vislobokova 2012).
 |
| Distribution of M. giganteus sites recently compiled by Lister and Stuart (2019). Note the relatively limited latitudinal range. |
Since the mid-1800s scholars have discussed where Megaloceros fits within deer phylogeny. Although firmly placed within Cervinae - the Old World deer clade - opinions differ over which extant deer are its closest relatives: Cervus (e.g. red deer, wapiti, sika deer - Kuehn et al. 2005) or Dama (fallow deer - Lister 1994; Geist 1999; Lister et al. 2005; Hughes 2005). The weight of morphological and DNA evidence for the Dama link is probably heavier at present, although this only resolves part of our struggles: how Megaloceros is related to other extinct deer is also uncertain. The composition, and even existence of a Megaloceros-line clade, Megacerini, is currently debated, as is whether Dama should be regarded as the last surviving megacerine (see Vislobokova 2012 for a review).
Extreme deer, extreme speed
Prehistoric animals tend to become popular because they represent some kind of biological extreme - the biggest, the heaviest and so on. It's curious, therefore, that the running capacity of Megaloceros is neither widely remarked upon nor commonly depicted in palaeoartworks. A wealth of anatomical data shows that Megaloceros was among the most cursorial of all deer - a species adapted for running far and long over wide, open ground (Geist 1999; Janis et al. 2012). Deer exhibit a range of running styles but Megaloceros seems to have been most suited to long-distance galloping with low limb ground clearance, in contrast to species which bound at height over rough terrain or high-step at speed through dense vegetation. Reindeer and wapiti are similarly adapted to long-distance running, though neither is comparable in cursorial adaptation to Megaloceros. Along with cursorial limb proportions, Megaloceros also has an evident muscle bulk consistent with running habits, highly 'hinged' shoulders permitting an enhanced forelimb stride length, and an expanded trunk volume for an enlarged heart and lungs (Geist 1999). Relatively small hooves indicate a preference running over firm ground. I'm not aware of any specific estimates of Megaloceros running speed, but Geist (1999) considered Megaloceros comparable to the fastest living deer - perhaps 80 kph (50 mph) - and assumed similarly commendable levels of stamina. The increased bulk of Megaloceros would have made for slower acceleration but, once at cruising speed, it might have been capable of running for hours and hours without stopping.
 |
| Many aspects of Megaloceros osteology - from limb proportions to chest volume, antler configuration and knee morphology - are consistent with a strongly cursorial lifestyle. It's ironic that this energetic, fast-running deer is often depicted in static poses in palaeoart when dynamic and active compositions typify the genre as a whole. Image by James St. John, from Wikimedia Commons, CC-BY 2.0. |
For Geist (1999), a running lifestyle is integral to explaining the development of Megaloceros size and antler spread. It stands to reason that the largest deer will also be the fastest and fittest, and antler size is a direct, honest reflection of stag health. These factors alone could have promoted the evolution of enormous antlers in a large cervid living in uncluttered habitats, but cursoriality encourages antler size in other ways too. For mammals, a consequence of evolving fast running is that juveniles have to be strong and coordinated enough to keep up with their parents, especially if running is critical to escaping danger. Megaloceros fawns must thus have also been capable runners from a very early age, requiring their parents to not only give birth to highly developed, precocial offspring but also supply them with milk rich enough to sustain long-distance, high-velocity running. Lo and behold, studies show that antler size correlates not only with offspring health and size, but also the amount of fat and nutrients in milk (Geist 1999). Put together, these factors mean Megaloceros wasn't a fast-running deer that happened to have huge antlers: its antlers were a direct consequence of its hyper-cursorial lifestyle.
But seriously though, those antlers
As is already evident, it's difficult to write about Megaloceros without frequent mention of its headgear, so let's tackle that topic head-on. There's a lot more to talk about here than just size. Antler structure, function and history of interpretation are also fascinating. Megaloceros antlers are extremely long - up to 1.7 m each - and broaden into great palms in their distal regions, with huge tines erupting from the burr, beam and palm margins. The first tine, which emerges just above the antler base, is broadened to differing extents in different subspecies, and is especially large in early representatives of the lineage (Lister 1994). This likely served as some sort of eye protector during combat (see below).
The size and elaboration of M. giganteus antlers placed it at the heart of a historic discussion about the fitness, evolution and extinction of fossil animals. M. giganteus stags were considered examples par excellence of orthogenic evolution in the late 19th and early 20th century when, as reviewed by Gould (1974), it was viewed as a species that had evolved itself into a corner: the result of a runaway, one-way evolutionary process that encouraged the creation of vastly oversized and biologically untenable antlers. Eventually, it was suggested, the antlers became so large and heavy that their owners were forever being caught in vegetation, mired in mud and bogs, or even suffered catastrophic brain haemorrhages caused by redirected blood flow from antler velvet. Extinction was inevitable for such sorry creatures.
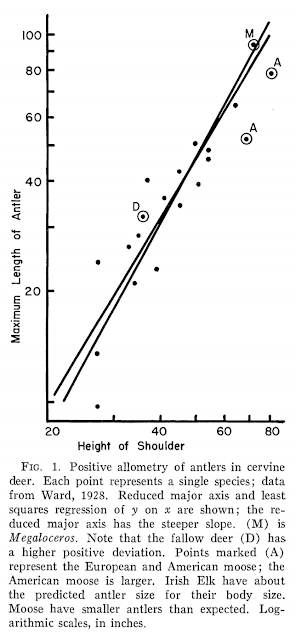
Of course, even an elementary grasp of modern principles of natural selection shows these ideas as naive, quaint and totally wrong - there is no conceivable mechanism through which species can evolve structures that are so hazardous to their health. But it wasn't until the 1930s that a sensible alternative explanation for Megaloceros antler size was proposed (positive allometry), and we waited until the 1970s for someone to actually test whether Megaloceros antlers were actually oversized, cumbersome organs or consistent in proportion to what we'd expect from living deer. This study came in the form of Stephen J. Gould's famous 1974 paper "The origin and function of 'bizarre' structures: antler size and skull size in the 'Irish Elk,' Megaloceros giganteus", which demonstrated that Megaloceros antler dimensions were not only consistent with those predicted from living deer, but that their evolution was explainable through regular old sexual selection - no crazy, uncontrolled evolutionary mechanism required. Further work has shown that, relative to body size, Megaloceros antlers were no heavier than those of fallow deer and, indeed, were actually lighter than those of reindeer (Geist 1999). Gould's results were an important grounding of Megaloceros in the wider context of deer biology. Rather than seeing it as a weird, dead-end outlier, Gould showed that Megaloceros represented the known limit of antlers operating as 'honest' signals of stag health and virility. Their size is a predictable outcome of cervine evolution should species develop into large-bodied, open-country specialists.
Furthermore, ideas that Megaloceros antlers were somehow evolving out of control ignores nuances of their structure and evolution. There's no doubt that - as with all stags - giant deer males were physiologically stressed by antler production. Even assuming development occurred over several months, centimetres of growth were required each day and it's doubted that enough calcium and phosphate could be processed directly from their food fast enough to meet demand (Moen et al. 1999). Megaloceros responded with the standard cervid adaptation of borrowing bony material from its skeleton, and physiological models suggest up to 80% of their available resorbable calcium was required to achieve a full antler spread - about twice that of moose (Moen et al. 1999). Assuming access to suitable plants, these resorbed minerals could be replaced in time to rebuild a strong, durable skeleton for the rut, but osteoporosis remained a risk for stags unable to recapture that material. It's thus unsurprising that Megaloceros antlers are, on average, not quite as solid as those of other deer. All antlers are a blend of compact bone (very dense and heavy, equating to a high mineral cost) and a spongier component (lighter and less dense, so lower mineral cost), and Megaloceros evolved a higher ratio of lower-density bone to better manage the physiological demands of antler growth. Even this was not enough to prevent their antlers from becoming a burden at times, however: average antler size is seen to reduce on occasion throughout their evolutionary history. These responses are the exact opposite of what we'd expect in an orthogenic 'runaway evolution' model, and are evidence of how ordinary selection pressures were keeping Megaloceros anatomy in step with environmental conditions.
 |
| Megaloceros antlers were exceptional communication devices, appearing enormous from multiple angles, and especially so from the front. Unlike other deer, Megaloceros would not have to pose to show off its antler palms: they were unmissable however you saw it. Screengrab of a 3D scan by National Museums Scotland that is loads of fun to play with (and forms a useful reference for artists!). |
The relatively low mineral value and size of Megaloceros antlers has led to discussions about their function: were they used for visual communication only? Palmate antler regions, which occur today in species like fallow deer and moose, are thought to serve primarily in communication, and it stands to reason that this applied to Megaloceros too. But while living palmate stags use head and neck motion to draw attention to their antlers (Gould 1974; Geist 1999), Megaloceros headgear was rotated in such a way that stags could exhibit their huge palms by simply standing still. This precluded the need to wave 40 kg of bone around for days on end, which was probably terrific news for the neck and head tissues of Megaloceros stags (Gould 1974). This said, Megaloceros skulls, necks and shoulders were heavily augmented to support their antlers (the skull roof was 30 mm thick! - Lister 1994) and it's probable that they were capable of moving them around with speed and precision, perhaps even violently. Although some authors (Gould 1974) have regarded Megaloceros stags as incapable of wrestling with one another, studies of their antler histology and stress distribution suggest such matches were possible (Lister 1994; Klinkhamer et al. 2019). The effects of clashing antlers have yet to be modelled - this being fraught with variables that are difficult to estimate at present - but Megaloceros antlers perform well under pushing and twisting regimes provided the stresses are primarily experienced in their proximal regions. They appear to have been especially resistant to rotational forces, and we might imagine stags locking their antlers together at close range, faces low to the ground, shoving and twisting each other to the floor (Lister 1994). Their antlers are so long that forces incurred at their tips might have been amplified to potentially bone-snapping levels however, so it's possible Megaloceros may have avoided especially violent, unpredictable fighting styles. Again, this is consistent with trends seen across deer: as stags become larger, and species become more sociable, their fights generally become more ritualised and lower risk. Megaloceros may be the ultimate example of this correlation (Geist 1999).
Life appearance
 |
| Charles Knight's 1906 take on Megaloceros in a traditional, red deer/wapiti form. Cervus-like reconstructions have typified this genus since at least the 1850s, when Waterhouse Hawkins reconstructed Megaloceros for the Crystal Palace Park. Image from Wikimedia Commons, in public domain. |
 |
| One of the most informative Palaeolithic artworks of Megaloceros is featured in the Megaloceros Gallery in France's famous Chauvet Cave. Unlike other examples, it seems to show shaded areas that could reflect darker body regions, similar to the banded Coelodonta depicted on the opposite wall. A second Megaloceros is featured a little further in, also showing a dark neck band. Alas, other Megaloceros panel drawings lack similar details, precluding determination of their reliability. Cropped from Google Arts and Culture - make sure to check out the Megaloceros Gallery in 3D! |
The challenges of interpreting Megaloceros colouration from cave art alone are many. Opinions differ on how many panel drawings we have - perhaps up to 40, but of which maybe only 15 or 16 are confidently identified as M. giganteus. Most are body outlines without details of colours or patterns and, to my knowledge, only 4-5 pieces feature those dark humps and body stripes. This is enough to assume that they are recording something about Megaloceros life appearance, but what, exactly, might that be? Are the lines radiating from the shoulder humps stripes, or something else, like the boundaries between colour regions? Are those simpler 'body outline' artworks actually depicting Megaloceros with uniform colouration? Do these differences reflect seasonal or regional colour variation, or else varying artistic approaches? The question of how literally we should take Palaeolithic depictions is not new, and it doesn't have a straightforward answer (Guthrie 2006).
 |
| Select Palaeolithic art of Megaloceros as interpreted by Guthrie (2006) - arrows indicate features of interest such as shoulder humps, expanded laryngeal regions etc. Note the absence of obvious colour and pattern data in most depictions - what significance, if any, might that have? This compilation of Guthrie's work is swiped from Tetrapod Zoology. |
Demise and extinction
As is always the case with a well-studied species, there's much more we could say about Megaloceros. We are still learning about its palaeobiogeography, behaviour, evolution and extinction from new discoveries and detailed examination of archived specimens. Digging into the literature on these animals has proven genuinely fascinating. As someone used to studying Mesozoic reptiles, the amount of information and insight we have on this species is almost overwhelming, and it seems that understanding its palaeobiology is challenged more by having to consider so much data rather than, as I'm more accustomed to, having too little information to draw meaningful conclusions. And yet, despite an excellent fossil record and centuries of study, some major aspects of its biology remain poorly understood or contested. Food for thought, indeed, about our capacity to interpret fossil tetrapods from more typical palaeontological datasets.
Enjoy these insights into palaeoart, fossil animal biology and occasional reviews of palaeo media? Support this blog for $1 a month and get free stuff!
This blog is sponsored through Patreon, the site where you can help online content creators make a living. If you enjoy my content, please consider donating $1 a month to help fund my work. $1 might seem like a trivial amount, but if every reader pitched that amount I could work on these articles and their artwork full time. In return, you'll get access to my exclusive Patreon content: regular updates on upcoming books, papers, paintings and exhibitions. Plus, you get free stuff - prints, high-quality images for printing, books, competitions - as my way of thanking you for your support. As always, huge thanks to everyone who already sponsors my work!
References
- Dussault, C., Ouellet, J. P., Courtois, R., Huot, J., Breton, L., & Larochelle, J. (2004). Behavioural responses of moose to thermal conditions in the boreal forest. Ecoscience, 11(3), 321-328.
- Geist, V. (1999). Deer of the World. Swan Hill Press, Shrewsbury.
- Gould, S. J. (1974). The origin and function of 'bizarre' structures: antler size and skull size in the 'Irish Elk,' Megaloceros giganteus. Evolution, 191-220.
- Guthrie, R. D. (2005). The Nature of Paleolithic Art. The University of Chicago Press.
- Hughes, S., Hayden, T. J., Douady, C. J., Tougard, C., Germonpré, M., Stuart, A., ... & Say, L. (2006). Molecular phylogeny of the extinct giant deer, Megaloceros giganteus. Molecular phylogenetics and evolution, 40(1), 285-291.
- Janis, C. M., Shoshitaishvili, B., Kambic, R., & Figueirido, B. (2012). On their knees: distal femur asymmetry in ungulates and its relationship to body size and locomotion. Journal of Vertebrate Paleontology, 32(2), 433-445.
- Klinkhamer, A. J., Woodley, N., Neenan, J. M., Parr, W. C., Clausen, P., Sánchez-Villagra, M. R., ... & Wroe, S. (2019). Head to head: the case for fighting behaviour in Megaloceros giganteus using finite-element analysis. Proceedings of the Royal Society B, 286(1912), 20191873.
- Knight, C. R. (1949). Prehistoric man: The great adventurer. Appleton-Century-Crofts.
- Kuehn, R., Ludt, C. J., Schroeder, W., & Rottmann, O. (2005). Molecular phylogeny of Megaloceros giganteus—the giant deer or just a giant red deer?. Zoological Science, 22(9), 1031-1044.
- Lister, A. M. (1994). The evolution of the giant deer, Megaloceros giganteus (Blumenbach). Zoological Journal of the Linnean Society, 112(1-2), 65-100.
- Lister, A. M., Edwards, C. J., Nock, D. A. W., Bunce, M., Van Pijlen, I. A., Bradley, D. G., ... & Barnes, I. (2005). The phylogenetic position of the ‘giant deer’ Megaloceros giganteus. Nature, 438(7069), 850-853.
- Lister, A. M., & Stuart, A. J. (2019). The extinction of the giant deer Megaloceros giganteus (Blumenbach): New radiocarbon evidence. Quaternary International, 500, 185-203.
- Moen, R. A., Pastor, J., & Cohen, Y. (1999). Antler growth and extinction of Irish elk. Evolutionary Ecology Research, 1(2), 235-249.
- van Beest, F. M., & Milner, J. M. (2013). Behavioural responses to thermal conditions affect seasonal mass change in a heat-sensitive northern ungulate. PloS one, 8(6), e65972.
- Vislobokova, I. A. (2012). Giant deer: origin, evolution, role in the biosphere. Paleontological Journal, 46(7), 643-775.













![[page image]](http://images.library.wisc.edu/HistSciTech/EFacs/CompAnat/0009/M/PLATEVI.jpg)
![[page image]](http://images.library.wisc.edu/HistSciTech/EFacs/CompAnat/0012/M/PLATEIX.jpg)
![[page image]](http://images.library.wisc.edu/HistSciTech/EFacs/CompAnat/0008/M/PLATEV.jpg)







